
This past summer, as the Carleton University Harry Reid Cox Intern at the Canadian Museum of Nature, I worked with museum researcher Aaron Lussier, Ph.D., to identify how common elements, such as silicon and iron, combine to make up the structures of large numbers of minerals.
The ultimate goal of this ongoing research is to determine why certain types of crystal structures are more common than others. Is it because they are more stable, or is it because of other factors?
This is a big question in mineral sciences. Believe it or not, we still don’t really understand why most minerals exist with the shape, form, atomic structures, and chemical compositions that they have.
So, the origins of crystal structure is a huge gap in our understanding of how the Earth system works.
We do know that minerals have fascinating internal crystal structure.
Minerals are made up of atoms that are perfectly ordered, creating crystals. Even a crystal that is big enough to hold in your hand, for example one on display in the museum’s Earth Gallery consists of many trillions of atoms, each and every one arranged in a very precise location.
Interestingly, minerals that look unrelated can share common structural aspects. For instance, they may have silicon atoms arranged in sheets, or aluminum atoms arranged as part of a seemingly infinite chain (See Figure 1).
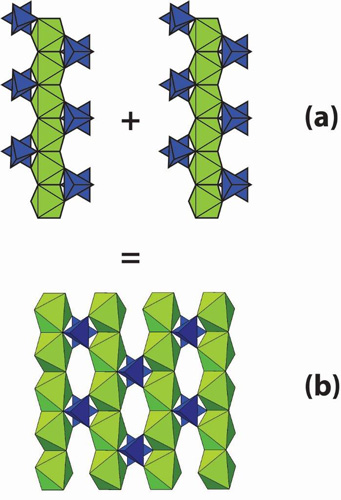
If we could answer the question of why minerals have a certain atomic structure, there would be many practical applications.
We could create better synthetic materials with specialized properties. We could engineer materials that interact predictably with local geology, or to aid in environmental remediation of harmful toxins, like lead and mercury, and even for the disposal of radioactive waste.
Just as with the diversity of mineral structures, the possibilities for their application could be endless!


