

Botany, Collections, Fieldwork
We’re Really Likin’ Lichens!

More Flat Plants Than I Can Imagine…Literally

From Field to Virtual Lab: Fossil Discovery in the Age of Pandemic

Room to Grow
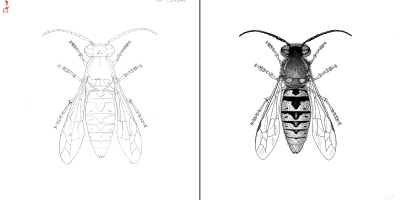
Tom Reaume: A Life Full of Possibilities

The Extraordinary Kinonge (Salmon) River and Its Unique Eastern Pearlshell Mussels

Arctic, Exhibitions, Uncategorized
Planet Ice Returns: The Show (Snow?) Must Go On

Flowers and Fiords: Botany in Agguttinni Territorial Park

